Octopus trial up for approval by MHRA and Research Ethics Committee
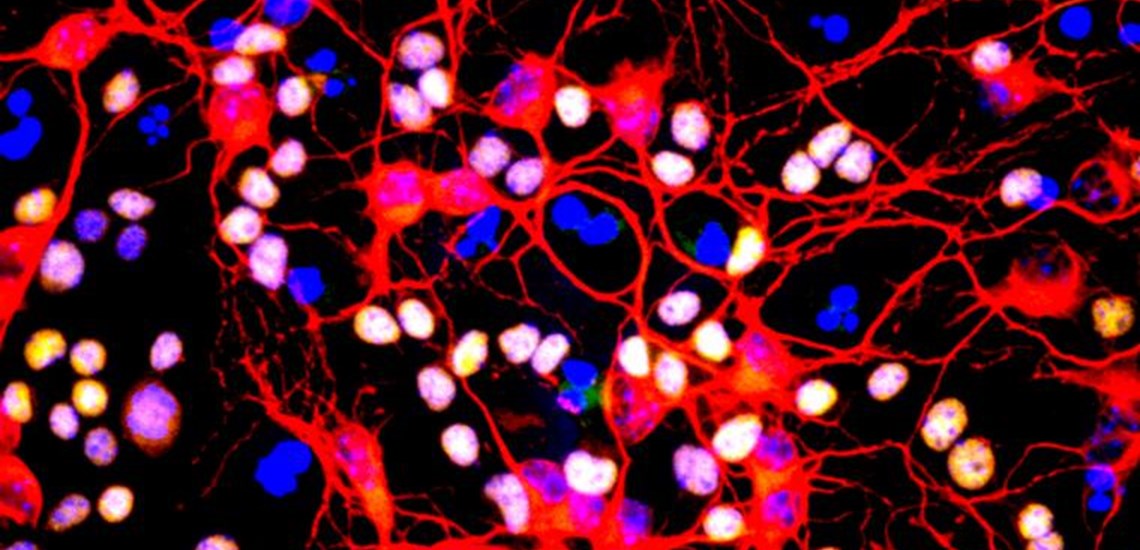
Myelination: oligodendrocyte engaging with neurites. By Nina Callard, via Wellcome Images. CC-BY-4.0
The trial team at UCL has recently submitted their application to the Medicines and Healthcare products Regulatory Agency (MHRA) and to a Research Ethics Committee. Gaining approval from both these bodies is mandatory for the trial to open to recruitment.
These organisations will review the application, raise any questions or request changes that they think are needed, and in due course give the green light for the trial to proceed. A response is expected within 60 days of submission.
While waiting, the team is continuing to set up the trial's database, sites, and processes that will be used to gather data from participants and manage the Octopus trial.

