We have now reached 326 participants randomised and 392 participants screened meaning we have recruited 87% of our goal for analysis stage 1 (375)!
Trial news
August Recruitment News!
We have now reached 302 participants randomised and 370 participants screened meaning we have recruited 81% of our goal for analysis stage 1 (375)!

OCTOPUS at MS Frontiers
Click here to read more about OCTOPUS at the MS Frontiers conference in July held at Liverpool.
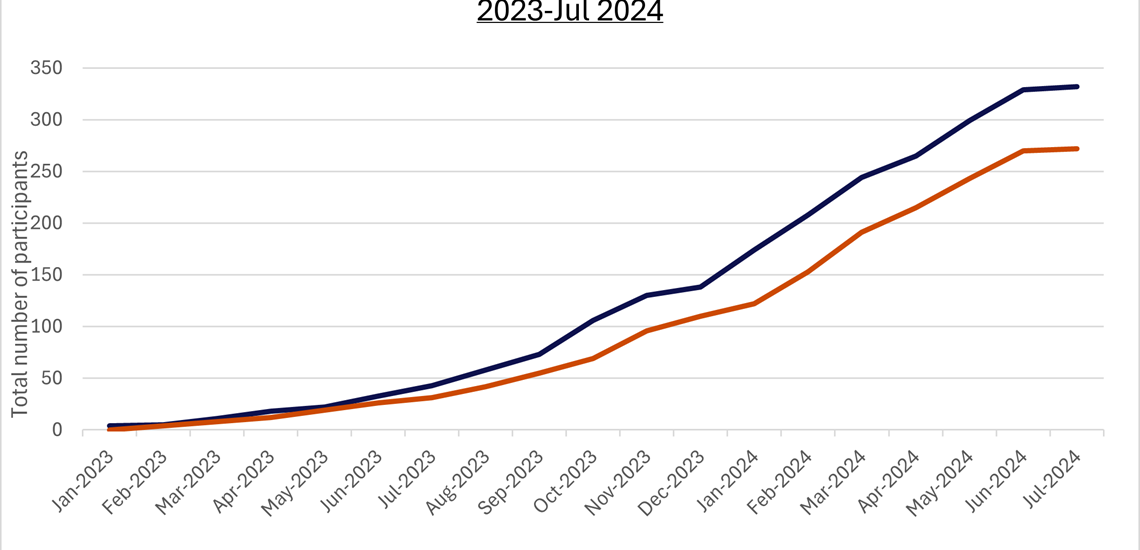
Recruitment News!
We have now reached 277 participants randomised and 375 participants screened meaning we have recruited 74% of our goal for analysis stage 1 (375)!

Asian MS: OCTOPUS and diversity in clinical trials webinar
Asian MS: OCTOPUS and diversity in clinical trials webinar

